Find the Number of Neutrons Po | Mathway. 84 84 protons Fill in the known values where N N represents the number of neutrons. 209 = 84+ N 209 = 84 + N Rewrite the equation as 84+N = 209 84 + N = 209. 84+N = 209 84 + N = 209 Move all terms not containing N N to the right side of the equation. N = 125 N = 125 find the number of neutrons po. How to Find the Number of Protons, Neutrons, and Electrons - wikiHow. 1 Get a periodic table of elements. The periodic table is a chart that organizes elements by their atomic structure. It is color-coded and assigns each element a unique 1 or 2-letter abbreviation. Other elemental information includes atomic weight and atomic number. [1] You can find a periodic table online or in a chemistry book.. How To Calculate The Number of Protons, Neutrons, and Electrons . find the number of neutrons po. We know that the mass number (A) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: p = 35 - 18 = 17, and therefore, the element is Cl The Number of Protons from Electrons For a neutral atom, the number of protons and the number of electrons are equal
Find the number of neutrons po
sanatorio chacabuco
. This is what makes the atom charge-free.. Polonium - Element information, properties and uses | Periodic Table. Element Polonium (Po), Group 16, Atomic Number 84, p-block, Mass [209]. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images.. How to Find the Number of Neutrons in an Atom: 11 Steps - wikiHow find the number of neutrons po. 1 Locate the element on the periodic table find the number of neutrons po


urotoin xr
. Number of protons and electrons are equal in an element. Hence, the number of protons and electrons will be 50. The number of neutrons will be 48.. Polonium - Mass Number - Neutron Number - Po - Periodic Table of Elements find the number of neutrons po. Mass numbers of typical isotopes of Polonium are 208-210 find the number of neutrons po. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. Neutron number plus atomic number equals atomic mass number: N+Z=A. The difference between the neutron number and the atomic number is known as the neutron excess: D .. Polonium - Periodic Table and Atomic Properties. Atomic Number - Protons, Electrons and Neutrons in Polonium. Polonium is a chemical element with atomic number 84 which means there are 84 protons in its nucleus.Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.. Polonium - Protons - Neutrons - Electrons - Electron Configuration find the number of neutrons po
πως να ζωγραφισω λουλουδια στον τοιχο
. Protons and Neutrons in Polonium. Polonium is a chemical element with atomic number 84 which means there are 84 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.. 11.4: Nuclear Decay - Chemistry LibreTexts. Allison Soult, Ph.D. (Department of Chemistry, University of Kentucky) 11.4: Nuclear Decay is shared under a CC BY-NC-SA 3.0 license and was authored, remixed, and/or curated by LibreTexts. Unstable nuclei spontaneously emit radiation in the form of particles and energy. This generally changes the number of protons and/or neutrons in the . find the number of neutrons po. Find the Number of Neutrons Pb | Mathway. Next, find the atomic number which is located above the element s symbol find the number of neutrons po. Since lead lead s atomic number is 82 82, Pb Pb has 82 82 protons. 82 82 protons. Fill in the known values where N N represents the number of neutrons. 207 = 82+ N 207 = 82 + N. Rewrite the equation as 82+N = 207 82 + N = 207. 82+N = 207 82 + N = 207. find the number of neutrons po. How many neutrons and protons in polonium 209? - Answers. More answers find the number of neutrons po. Polonium 209 has 84 electrons and protons and 125 neutrons. Polonium-210 has 84 protons and 126 neutrons find the number of neutrons po. Polonium-210 has 84 protons and 126 neutrons. Number of protons = Atomic .. 4.8: Isotopes - When the Number of Neutrons Varies. For all atoms with no charge, the number of electrons is equal to the number of protons. [text {number of electrons} = 19 nonumber ] The mass number, 40, is the sum of the protons and the neutrons. To find the number of neutrons, subtract the number of protons from the mass number.. Find the Number of Neutrons Cd | Mathway. Since cadmium cadmium s atomic number is 48 48, Cd Cd has 48 48 protons. Fill in the known values where N N represents the number of neutrons. Rewrite the equation as 48+N = 112 48 + N = 112. Move all terms not containing N N to the right side of the equation.. 1.2: Atomic Structure - The Nucleus - Chemistry LibreTexts. Each isotope of a given element has the same atomic number but a different mass number (A), which is the sum of the numbers of protons and neutrons. The relative masses of atoms are reported using the atomic mass unit ( amu ), which is defined as one-twelfth of the mass of one atom of carbon-12, with 6 protons, 6 neutrons, and 6 electrons. find the number of neutrons po. Solved 2.49 (B) Applications Calculate the number of - Chegg. Question: 2.49 (B) Applications Calculate the number of protons, neutrons, and electrons in: 2.28 Calculate the number of protons, neutrons, and electrons in: Light, 2.29 An atom has nine protons, ten neutrons, and nine elec- trons find the number of neutrons po
城市花園酒店
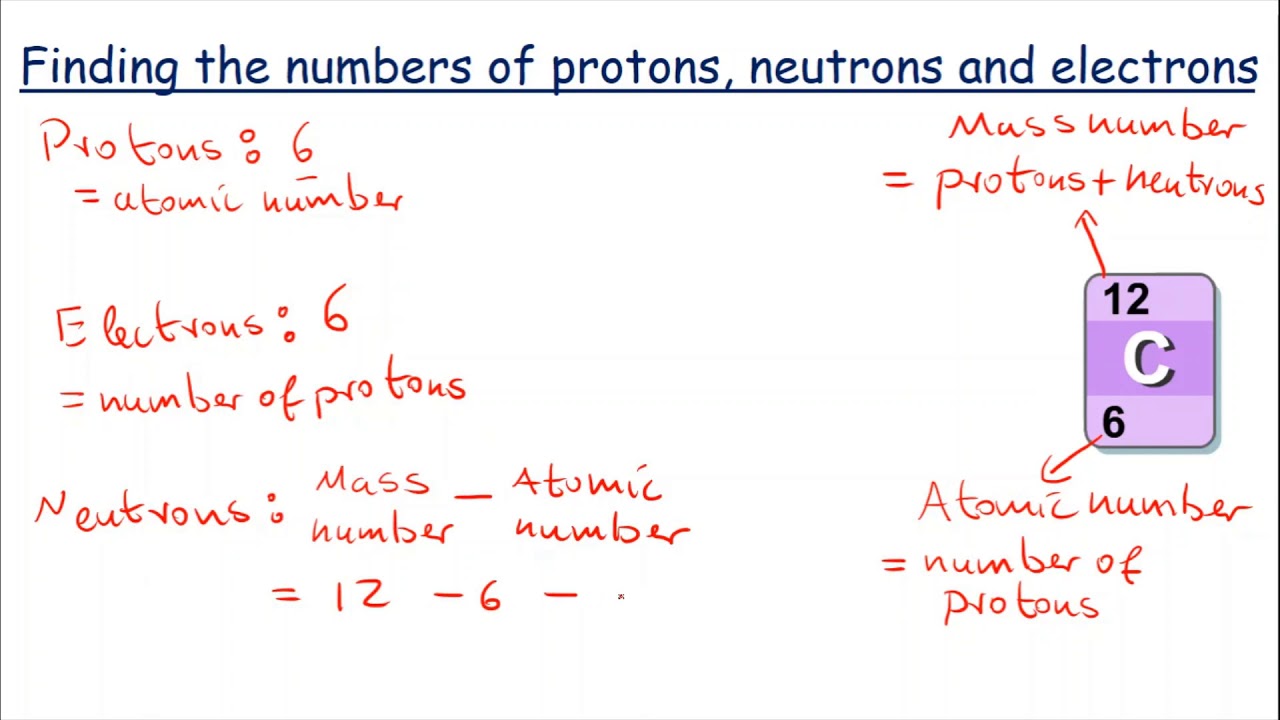
. Structure of the Atom - Division of Chemical Education, Purdue University. 1.008665 amu. The number of protons, neutrons, and electrons in an atom can be determined from a set of simple rules. The number of protons in the nucleus of the atom is equal to the atomic number ( Z ). The number of electrons in a neutral atom is equal to the number of protons. The mass number of the atom ( M) is equal to the sum of the .. How To Calculate The Number of Protons, Neutrons, and Electrons .
demans hastasının ölüm belirtileri
. This chemistry video tutorial explains how to calculate the number of protons, neutrons, and electrons in an atom or in an ion find the number of neutrons po. It also explains the differe.. Doubt Solutions - Maths, Science, CBSE, NCERT, IIT JEE, NEET. Calculate number of electrons present in 3.5 g of PO :-02:20. View Solution. Calculate the total number of electrons present in 1.4 g of dinitrogen gas. Text Solution. Calculate number of neutrons present in 12 X 10^25 atoms of oxygen (8. 03:16. If mass of one atom is 3.32 xx 10^(-23)g, then calculate number of nuc.. How can you determine the number of neutrons in an atom?. Since we know the atomic number of Cl is 17, we can work out that Cl-35 has 18 neutrons, and Cl-37 has 20 neutrons find the number of neutrons po. Remember - the atomic number of an element is constant for all isotopes, since it is the number of protons that actually defines which element an atom is.. PDF Polonium-210 Fact Sheet - Health Physics Society. Po) find the number of neutrons po. With a find the number of neutrons po. half-life find the number of neutrons po. of 138 days, it decays to stable lead-206 by the emission of an . alpha particle . A form of a chemical element that has a different number of neutrons. Carbon-12 and carbon-14 are two isotopes of carbon and each has 6 protons, but carbon-12 has 6 neutrons and carbon-14 has 8 neutrons. .. Calculate the total number of electrons , protons and neutrons in PO . find the number of neutrons po. Atomic number of aluminum is 13 and mass number is 27. Calculate the number of electrons, protons and neutrons inits atom. Represent the ion of this element.. Answered: 270, How many protons and neutrons are… | bartleby. Match these by placing the correct notation in the appropriate blank. 3467Se3367As3567Br3672Kr a. Contains 33 neutrons b. Contains greatest number of neutrons c. Contains equal number of protons and neutrons d. Contains the same number of neutrons as there are protons in As-67. 2.3: Atomic Structure and Symbolism - Chemistry LibreTexts find the number of neutrons po. The mass of one atom is usually expressed in atomic mass units (amu), which is referred to as the atomic mass. An amu is defined as exactly 1/12 1 / 12 of the mass of a carbon-12 atom and is equal to 1.6605 × × 10 −24 g. Protons are relatively heavy particles with a charge of 1+ and a mass of 1.0073 amu. find the number of neutrons po. Introduction to Isotopes | Department of Chemistry - Texas A&M University. If you know the number of protons, neutrons, and electrons in an element, it is a simple matter to calculate the mass of that element. This brings us to two common terms used in chemistry, atomic number and mass number. PO Box 30012 College Station, TX 77842-3012. Shipping Address. Department of Chemistry Texas A&M University 580 Ross . find the number of neutrons po. Isotopes II - Chemistry LibreTexts find the number of neutrons po. Because the atomic number of every carbon atom is 6, carbon-12 has 6 protons and therefore 6 electrons. To find the number of neutrons, we subtract the number of protons from the mass number: 12 - 6 = 6 neutrons. The data in Table 1-1 can be used to calculate the total mass of these particles: Protons: 6 x 1.00728 amu = 6.04368 u. How to find Protons, Neutrons, and Electrons? - EnthuZiastic. The number of neutrons in an atom can be determined by subtracting the atomic number from the mass number: Therefore, the relationship between A and Z is: N = A - Z. 2. Sample Problems to find Protons, Neutrons, and Electrons. Here are a few examples of problems to calculate the number of protons, electrons, and neutrons in an atom: 2.1 How .. Polonium (Po) - Periodic Table (Element Information & More) find the number of neutrons po. 1st shell can hold 2 electrons. 2nd shell can hold 8 electrons. 3rd shell can hold 18 electrons find the number of neutrons po. 4th shell can hold 32 electrons. Now the atomic number of Polonium (Po) is. Hence the polonium element has electrons arrangement 2, 8, 18, 32, 18, 6 find the number of neutrons po. This electron arrangement indicates that the outermost orbit of polonium element (Po) has 6 electrons.. Doubt Solutions - Maths, Science, CBSE, NCERT, IIT JEE, NEET find the number of neutrons po. Arrange the following statements in a sequence which involves the calculation of the atomic number and mass number for an atom of an element with 15 electrons and 16 neutrons. (1) A = Number of protons + Number of neutrons A = Z + Number of neutrons A = 15 + 16 = 31 (2) Number of protons and number of electrons are equal in a neutral atom.. Solved 1. State the number of neutrons and protons in each - Chegg. Question: 1. State the number of neutrons and protons in each of the following nuclei , label each as radioactive or radioisotope or none a.2 H: b.12 C6 c. P31 15 : d.197 Au 79 2 find the number of neutrons po. The three types of radioactive emissions are called alpha (a), beta (B) and gamma (Y) radiation find the number of neutrons po. Complete the table below with the correct information about each type.. How Many Protons Neutrons and Electrons are in Calcium - Unacademy find the number of neutrons po. The mass number or atomic mass of Calcium is known to be 40. The number of protons as well as number of electrons is equal to the atomic number of an atom. Therefore, the number of protons in calcium are 20. The number of electrons in calcium are also 20
cserépedény
. Along with this, the number of neutrons in calcium are also 20.. Calculate number of electrons present in 9.5 g of PO^3 - 4 - Toppr find the number of neutrons po. Get the Free Answr app. Click a picture with our app and get instant verified solutions find the number of neutrons po. Click here👆to get an answer to your question ️ 30 alculate number of electrons present in 9.5 g of PO (b) 5 NA (c) 0.1NA of atom in 126 amy of HÑO, ? (d) 4.7 NA.. 4.5: Elements- Defined by Their Number of Protons. The mass number ( (A)) of an atom is the total number of protons and neutrons in its nucleus. The mass of the atom is a unit called the atomic mass unit (left ( text {amu} right))
kojic gluta papaya arbutin soap
. One atomic mass unit is the mass of a proton, or about (1.67 times 10^ {-27}) kilograms, which is an extremely small mass.. atomic structure periodic table | 188 plays | Quizizz. Group 18. Multiple Choice. 30 seconds. 1 pt find the number of neutrons po. The atomic number of krypton is 36 find the number of neutrons po. If the mass number of a krypton atom is 84, which answer shows the number of subatomic particles inside and outside the nucleus of the krypton atom? Number of Particles Inside Nucleus 36. Number of Particles Outside Nucleus 36.. Nuclear Magic Numbers - Chemistry LibreTexts. Calculate the total number of nucleons (protons and neutrons) in the nuclide. If the number of nucleons is even, there is a good chance it is stable. Are there a magic number of protons or neutrons? 2,8,20,28,50,82,114 (protons), 126 (neutrons), 184 (neutrons) are particularly stable in nuclei.. 1.1: Atomic Structure - The Nucleus - Chemistry LibreTexts. Each isotope of a given element has the same atomic number but a different mass number (A), which is the sum of the numbers of protons and neutrons. The relative masses of atoms are reported using the atomic mass unit ( amu ), which is defined as one-twelfth of the mass of one atom of carbon-12, with 6 protons, 6 neutrons, and 6 electrons.
lomiteria
. Solved 2.49 (B) Applications Calculate the number of - Chegg. Expert Answer find the number of neutrons po. 2.27 a) Ba has 56 protons, 56 electrons and 136 - 56 = 80 neutrons. b) Po has 84 protons, 84 electrons and 290 - 84 = 125 neutrons. 2.28 a) Cl has 17 prot …. 2.49 (B) Applications Calculate the number of protons, neutrons, and electrons in: 2.28 Calculate the number of protons, neutrons, and electrons in: Light, 2.29 An atom .. Protons, Neutrons, Electrons for Neptunium (Np, Np3+). Number of neutrons through isotopes of neptunium. Of the 25 radioisotopes of neptunium, the longest-lived radioisotope is neptunium-237 with a half-life of 2.144×10 6 years. The next longest-lived neptunium isotope is neptunium-236 with a half-life of 1.54×10 5 years and neptunium-235 with a half-life of 396.1 days find the number of neutrons po. How many protons, neutrons and electrons does a neptunium ion(Np 3+) have?. 4.7: Isotopes and Mass Numbers - Chemistry LibreTexts. This is because the mass of the proton and neutron are each about 1 amu, while the mass of the electron is very small in comparison find the number of neutrons po. mass number(A) = number of protons + number of neutrons (4.7.1) (4.7.1) mass number ( A) = number of protons + number of neutrons. Consider oxygen, which has an atomic number ( Z Z) of 8.. Atom Calculator. Alternatively, you can also calculate the atomic number, atomic mass, and charge. Choose your element. Lets assume that it is the sulfide anion. Find the numbers of protons, neutrons, and electrons. They are equal to 16, 16, and 18, respectively. Calculate atomic number, atomic mass, and charge by using mathematical expressions (4-6): Z = 16 (4). Polonium - Atomic Number - Atomic Mass - Density of Polonium. Atomic Number of Polonium. Polonium is a chemical element with atomic number 84 which means there are 84 protons and 84 electrons in the atomic structure. The chemical symbol for Polonium is Po. Since the number of electrons is responsible for the chemical behavior of atoms, the atomic number identifies the various chemical elements. How does the atomic number determine the chemical behavior . find the number of neutrons po. 3.5: Isotopes - Chemistry LibreTexts find the number of neutrons po. The isotopes of an element differ only in their atomic mass, which is given by the mass number (A), the sum of the numbers of protons and neutrons find the number of neutrons po. (CC BY-NS-SA; anonymous by request) The element carbon ( ce {C}) has an atomic number of 6, which means that all neutral carbon atoms contain 6 protons and 6 electrons. find the number of neutrons po. How many protons and neutrons in 209Po? - Answers find the number of neutrons po. Copy. Atomic number of Po is 84. Hence there are 84 protons. Number of neutrons for 209Po = 209 - 84 = 125 find the number of neutrons po. This answer is: Wiki User. ∙ 12y ago. Copy. 126.. Polonium - Wikipedia. Not to be confused with Polonium-210 Polonium is a chemical element; it has symbol Po and atomic number 84. A rare and highly radioactive metal with no stable isotopes, find the number of neutrons po. at a rate of 93 neutrons per million alpha particles find the number of neutrons po. Po-BeO mixtures are used as passive neutron sources with a gamma-ray-to-neutron production ratio of 1.13 ± 0.05, .. How many neutrons are in Pb-210? - Answers. So if you can locate a Periodic Table, you can see that leads atomic number of 82, which means it has 82 protons. If you do a little subtraction (210-82), you can find the number of neutrons in . find the number of neutrons po. Answered: 11) To calculate the number of neutrons… | bartleby. 11) To calculate the number of neutrons in an atom, you would subtract A) mass number from atomic number B) atomic number from electron number C) atomic number from mass number 12) What is the correct notation for a particle which has 17 protons, 18 neturals and 18 electrons? A) ECI- B) CI- c) #CI. Problem 2.101P: 2-101 Complete the following .. 4.5: Elements- Defined by Their Numbers of Protons. Figure 4.5.1 4.5 find the number of neutrons po. 1: It is difficult to find qualities that differ between each element, and to distinguish one element from another. Each element, however, does have a unique number of protons. Sulfur has 16 protons, silicon has 14 protons, and gold has 79 protons.. 4.4: Protons, Neutrons, and Electrons - Chemistry LibreTexts. Thats why the neutrons in the table below are labeled (n^0). The zero stands for "zero charge". A neutron is slightly more massive than a proton (see below) and has about the same diameter. Neutrons are found in all atoms (except for most atoms of hydrogen) and may be found bound together with other neutrons and protons in the atomic nucleus. find the number of neutrons po. Radon - Protons - Neutrons - Electrons - Electron Configuration. Protons and Neutrons in Radon. Radon is a chemical element with atomic number 86 which means there are 86 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.. Iron - Mass Number - Neutron Number - Fe - Periodic Table of Elements. Neutron Number and Mass Number of Iron find the number of neutrons po. Mass numbers of typical isotopes of Iron are 56; 57; 58. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N.Neutron number plus atomic number equals atomic mass number: N+Z=A.The difference between the neutron number and the atomic number is known as the neutron excess: D = N - Z .
. Answered: Part A Determine the number of protons… | bartleby find the number of neutrons po. Question. Please answer 14 Part B, C, and D